Nitrate: The Element
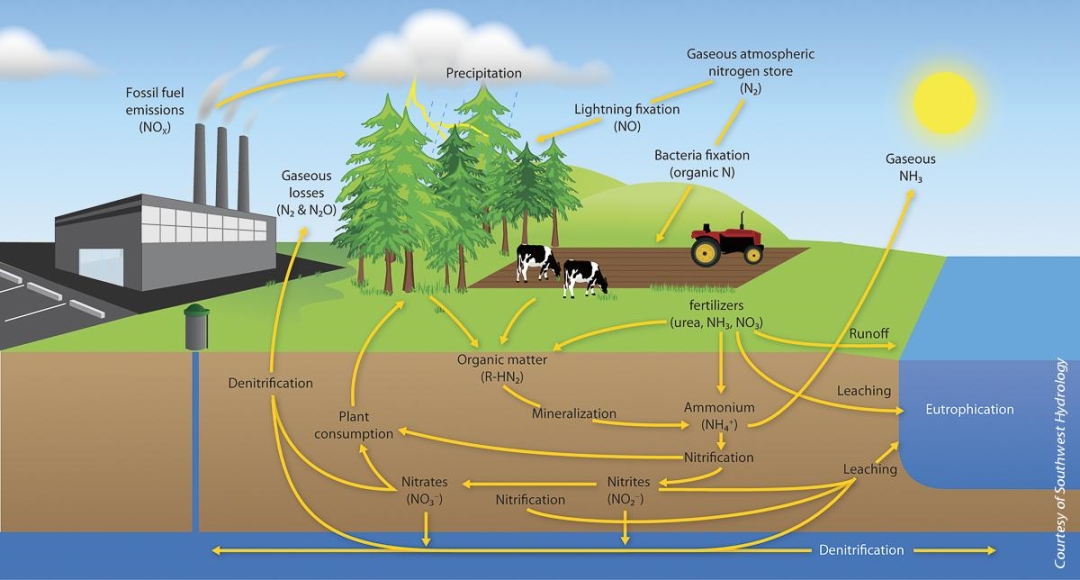
In the atmosphere, nitrogen gas (N2) is the primary form of nitrogen and is the most abundant element in the atmosphere where it comprises 78% of air 1. Nitrogen is found in many forms, the major ones being N2 (atmospheric nitrogen), N2O (nitrous oxide), NO (nitric oxide), NO2 (nitrite), NO3 (nitrate) and NH3 (ammonia). Some of these compounds are in gaseous phase and readily react with rain water, or surface water, to produce nitrate and ammonium ions. These ions become part of the soil layer, eventually permeating through into ground water aquifers creating a natural background concentration of nitrate, usually less than 2 milligrams per liter (mg/L)5. The most notable ion is nitrate, a hydrophilic anion. As nitrate passes through the soil, it can be converted back into atmospheric nitrogen (N2) through a process known as denitrification. This may take place in the soil by denitrifying bacteria that reduce nitrate 2. As conversion takes place, nitrogen is ejected back into the atmosphere as to complete the nitrogen cycle. Due to high solubility and weak retention by soil, nitrates are very mobile in soil, moving at approximately the same rate as water, and has a high potential to migrate to ground water. Because it does not volatilize, nitrate is likely to remain in water until consumed by plants or other organisms.
References
1 Haller, L., McCarthy, P., OBrian, T., Riehle, J., & Stuhldreher, T. (1999). Nitrate pollution of groundwater. Retrieved 3/20/2013 from http://www.reopure.com/nitratinfo.html
2 Denitrifying Bacteria. Encyclopedia Britannica. Retrieved 2/12/13 from http://www.britannica.com/EBchecked/topic/157733/denitrifying-bacteria